Abstract
Introduction: The most commonly used conditioning regimen for autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma (MM) is high-dose melphalan. However, conventional melphalan formulations can put patients at risk of potential propylene glycol (PG)-associated toxicities. In addition, the traditional melphalan formulations are unstable at room temperature, which prevents studying longer infusion schedules. The higher stability and potentially lower toxicity of PG-free (PGF) melphalan (Evomela) supports the evaluation of different doses and prolonged infusion schedules, in addition to the traditional 30-60 minute bolus doses.
Here, we report interim results of a trial designed to assess whether the characteristics of PGF melphalan allow for a higher dose or prolongation of infusion time, in order to increase the efficacy of melphalan in myeloma patients undergoing auto-HCT.
Methods: The primary objective of this two-stage phase I-II trial is to evaluate the optimal dose and schedule of PGF melphalan given as a single agent preparative regimen on day -2 before auto-HCT. The study enrolls adults with non-relapsed MM, a Karnofsky performance score ≥70%, who have received ≥2 cycles of initial systemic therapy and were within 2 to 12 months of their first induction. Participants are randomized (1:1) to two different infusion schedules (30-60 minute or 8-9 hour) using Evomela (2mg/ml) at one of two doses (200 or 225 mg/m 2). The first 3 patients in each schedule were treated at the dose of 200 mg/m 2. Since no DLT was observed, the dose was escalated to 225 mg/m 2. All patients will continue to receive 225 mg/m 2 in the absence of DLT. Disease response was assessed according to the International Myeloma Working Group uniform response criteria. Minimal residual disease (MRD) was measured by using multiparametric flow cytometry (10 -5) in the bone marrow at day-90 after transplant.
Secondary outcomes include incidence of treatment related mortality, rate of MRD, complete response at 90 days post auto-HCT, and progression-free and overall survival.
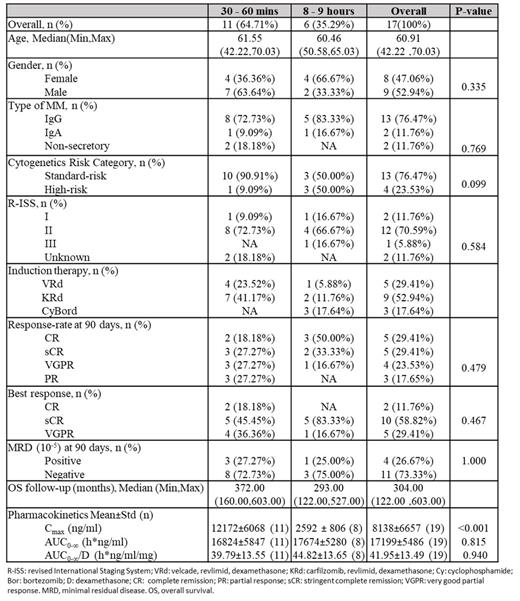
Results: To date (July 20,2021), 24 eligible patients have been randomized. Seventeen patients (47% female, mean age 59) are eligible for assessing response 90 days post-treatment, 11 (64.7%) in schedule 1 (30-60 mins) and 6 (35.3%) in schedule 2 (8-9 hrs). The overall response rate (partial response [PR] or better) is 100%, with 5(29.4%) stringent complete remissions (sCR), 5 (29.4%) complete remissions (CR), 4(23.5%) very good partial responses (VGPR), and 3(17.6%) partial remission (PR). The CR+sCR rate is 58.8%. Of the 15 patients eligible for MRD assessment, 11 (73.3%) in schedule 1 and 4 (26.7%) in schedule 2, 11/15 (73.3%) were MRD-negative and 8/15 (53.3%) were sCR/CR+MRD-negative 90 days post-treatment. The median follow-up is 11 months (95%CI:6.1-15.4) No patient has experienced disease progression or death.
Dose-limiting toxicity (DLT) is defined as grade 4 mucositis, or any grade 4 or 5 non-hematologic or non-infectious toxicity occurring within 30 days from the start of infusion. Twenty-one patients were evaluable for toxicity monitoring and no patient experienced >grade 3 adverse events with either schedule. Notably, no patient experienced ≥grade 3 esophagitis or oral mucositis. Diarrhea was the most frequent adverse event, and the incidence of grade 3 diarrhea was 18% in the short infusion arm and 43% with the longer infusion. No DLTs have been observed.
On pharmacokinetics analysis, the C max was highest in the short infusion arm (p<0.001). The area under the curve (AUC) 0-∞, including the dose-normalized AUC 0-∞ was lower in the short infusion arm; however, the differences were not statistically significant (p=0.815 and p=0.940, respectively).
Conclusions: Preliminary trial results have demonstrated that PGF melphalan, as a high-dose conditioning regimen for auto-HCT in patients with MM, has an acceptable safety profile (no >grade 3 events). The response rates, including the CR+MRD-negative rates are encouraging. The impact of bolus versus longer infusion schedule on outcomes will be assessed after the completion of the trial.
Mehta: Kadmon: Research Funding; CSLBehring: Research Funding; Syndax: Research Funding; Incyte: Research Funding. Popat: Bayer: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Lee: Genentech: Consultancy; Legend Biotech: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Sanofi: Consultancy; Takeda Pharmaceuticals: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Regeneron: Research Funding; Bristol Myers Squibb: Consultancy; Oncopetides: Consultancy; Karyopharm: Consultancy; Janssen: Consultancy, Research Funding; Celgene: Consultancy. Thomas: Acerta Pharma: Research Funding; Ascentage Pharma: Research Funding; BMS: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; X4 Pharma: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding. Patel: Pfizer: Consultancy; Janssen: Consultancy, Research Funding; BMS Celgene: Consultancy, Research Funding; Oncopeptides: Consultancy. Orlowski: Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, EcoR1 Capital LLC, Genzyme, GSK Biologicals, Janssen Biotech, Karyopharm Therapeutics, Inc., Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda P: Consultancy, Honoraria; CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Other: Clinical research funding; Asylia Therapeutics, Inc., BioTheryX, Inc., and Heidelberg Pharma, AG.: Other: Laboratory research funding; Asylia Therapeutics, Inc.: Current holder of individual stocks in a privately-held company, Patents & Royalties; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, Forma Therapeutics, Genzyme, GSK Biologicals, Janssen Biotech, Juno Therapeutics, Karyopharm Therapeutics, Inc., Kite Pharma, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, I: Membership on an entity's Board of Directors or advisory committees. Shpall: Takeda: Patents & Royalties; Affimed: Patents & Royalties; Adaptimmune: Consultancy; Magenta: Consultancy; Novartis: Consultancy; Bayer HealthCare Pharmaceuticals: Honoraria; Axio: Consultancy; Navan: Consultancy; Novartis: Honoraria; Magenta: Honoraria. Qazilbash: Bristol-Myers Squibb: Other: Advisory Board; Amgen: Research Funding; Oncopeptides: Other: Advisory Board; Angiocrine: Research Funding; NexImmune: Research Funding; Janssen: Research Funding; Biolline: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal